海泡石TiO2的制备及其对乙酰甲胺磷催化降解特性的研究
摘要本课题拟制备出一种海泡石/TiO2复合催化剂,以充分利用海泡石的吸附性能和TiO2的催化特性,达到降解有机磷农药的目的。试验首先以乙酰甲胺磷为吸附对象研究了海泡石的吸附性能;其次采用钛酸四丁酯水解法制备出TiO2催化剂并在超声协同的条件下对乙酰甲胺磷进行了降解;最后采用微波辅助溶胶-凝胶法制备出海泡石/TiO2复合催化剂,并探讨了温度、pH值、乙酰甲胺磷初始浓度、海泡石/TiO2复合催化剂用量、通气、H2O2、Fe2+等因素对海泡石/TiO2复合催化剂光催化降解的影响。主要结论如下:(1)海泡石的主要组成成分为SiO2和MgO,其质量分数分别为50.7%和24.3%,其平均颗粒直径为0.1...
相关推荐
-
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费

 2024-10-14 41
2024-10-14 41 -
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 53
2024-10-14 53 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(原卷版)VIP免费

 2024-10-14 39
2024-10-14 39 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(解析版)VIP免费

 2024-10-14 30
2024-10-14 30 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(原卷版)VIP免费
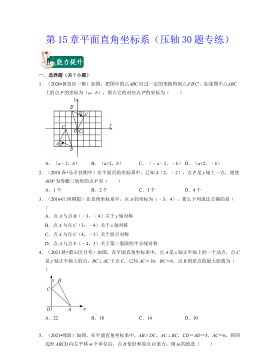
 2024-10-14 47
2024-10-14 47 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)VIP免费

 2024-10-14 44
2024-10-14 44 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)VIP免费

 2024-10-14 38
2024-10-14 38 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)VIP免费
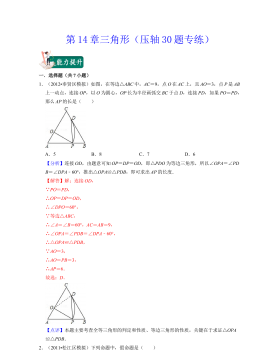
 2024-10-14 47
2024-10-14 47 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费
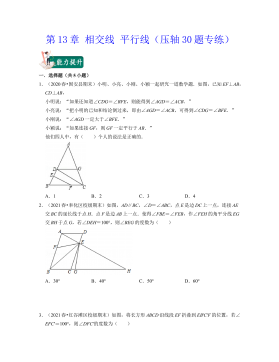
 2024-10-14 44
2024-10-14 44 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 44
2024-10-14 44
相关内容
-

七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分






