活性炭负载纳米零价铁的合成及对六氯苯的吸附研究
活性炭负载纳米零价铁的合成及对六氯苯的吸附研究摘要六氯苯(HCB)是12种持久性有机污染物之一,具有致癌、致畸和致突变效应,损伤肝脏,造成生长发育停滞,甚至可遗传性。治理含HCB的污染水体对环境保护和人类安全维护有重要意义。活性炭和纳米零价铁(nZVI)应用广泛,并能高效吸附降解有机物,降低或消除其毒性。本研究分别采用浸渍法和还原法合成活性炭负载nZVI(nZVI/AC),确定其最佳合成条件,并探讨该复合材料对以HCB为代表的有机物的去除效果及机理。表征和吸附平衡实验结果表明铁碳质量比、加热时间和乙醇投加量等因素对铁的负载过程有重要影响。活性炭负载纳米零价铁后,其比表面积和孔体积下降,降低程度...
相关推荐
-
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费

 2024-10-14 25
2024-10-14 25 -
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 28
2024-10-14 28 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(原卷版)VIP免费

 2024-10-14 27
2024-10-14 27 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(解析版)VIP免费

 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(原卷版)VIP免费
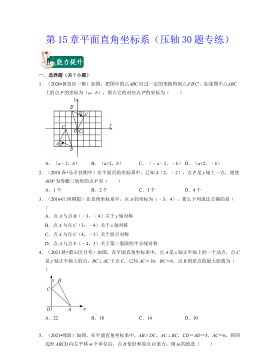
 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)VIP免费

 2024-10-14 27
2024-10-14 27 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)VIP免费

 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)VIP免费
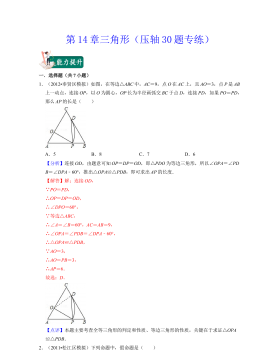
 2024-10-14 30
2024-10-14 30 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费
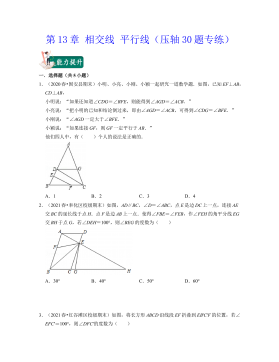
 2024-10-14 26
2024-10-14 26 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 22
2024-10-14 22
相关内容
-

七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分






