壳聚糖交联β- 环糊精吸附水中铬和镉的研究
摘要随着现代经济的迅猛发展,一些机械加工、化学化工、矿山开采等行业排放大量未经达标处理的重金属废水,造成了严重的水体重金属污染。环境水体中的重金属的存在状态及变化复杂,重金属无论采用什么工艺都不能被彻底降解,只有改变其状态从而将其从水中去除。本文研究了壳聚糖的改性方法,制成壳聚糖交联β-环糊精高聚物(CTS-CD),并将其应用于水中铬和镉的吸附去除研究。详细研究了CTS-CD对水中铬和镉的吸附性能和规律,探讨了吸附机理,研究了材料的再生和循环利用。以戊二醛作交联剂,将β-环糊精成功交联壳聚糖大分子链上,制得CTS-CD。研究了各合成因素对CTS-CD吸附去除铬和镉的影响,由此确定制备条件为:反...
相关推荐
-
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费

 2024-10-14 25
2024-10-14 25 -
七年级数学下册(易错30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 28
2024-10-14 28 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(原卷版)VIP免费

 2024-10-14 27
2024-10-14 27 -
七年级数学下册(易错30题专练)(沪教版)-第12章 实数(解析版)VIP免费

 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(原卷版)VIP免费
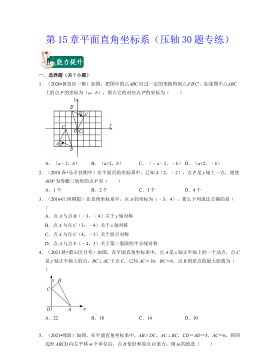
 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)VIP免费

 2024-10-14 27
2024-10-14 27 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)VIP免费

 2024-10-14 19
2024-10-14 19 -
七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)VIP免费
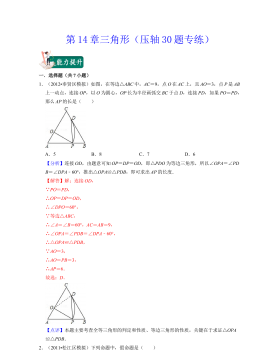
 2024-10-14 30
2024-10-14 30 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)VIP免费
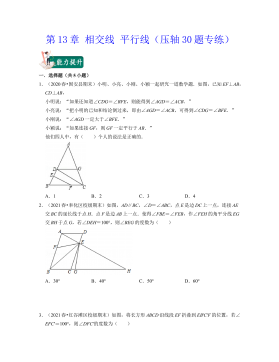
 2024-10-14 26
2024-10-14 26 -
七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)VIP免费

 2024-10-14 22
2024-10-14 22
相关内容
-

七年级数学下册(压轴30题专练)(沪教版)-第15章平面直角坐标系(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第14章三角形(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(原卷版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分
-

七年级数学下册(压轴30题专练)(沪教版)-第13章 相交线 平行线(解析版)
分类:中小学教育资料
时间:2024-10-14
标签:无
格式:DOCX
价格:15 积分






